Researchers from New Delhi and Pune, India, found out that melatonin, a hormone responsible for maintaining our biological clock can become a treatment option for Alzheimer’s Disease.
Melatonin is a hormone produced naturally by our body and is mostly known for its circadian rhythm role: it regulates your body clock, being reduced during the day and produced in high quantities at night, being attributed to the sleepiness at night and awareness on the morning. That said, it’s a molecule with various desirable features: most cells in the human body have receptors for it (hence they recognize it readily), it’s amphipathic (so it can penetrate virtually all cell membranes in the organism), it has good anti-oxidative properties, readily absorbed in the stomach and has a low toxicity; in fact, in Brazil it’s prescribed by some physicians as an alternative drug for insomnia.
It was found, though, that melatonin may also be a good candidate for Alzheimer’s Disease treatment, known to have few available treatments but no cure for now. Researches from the last two decades show that it may regulate the metabolization of Tau protein – a molecule linked to chronic mental disorders, such as Alzheimer’s Disease and Parkinson’s Disease.
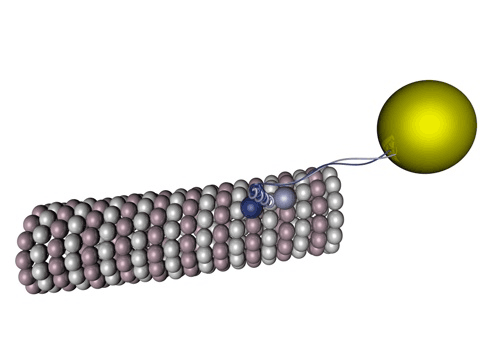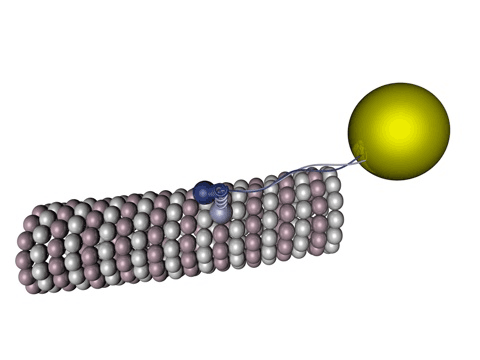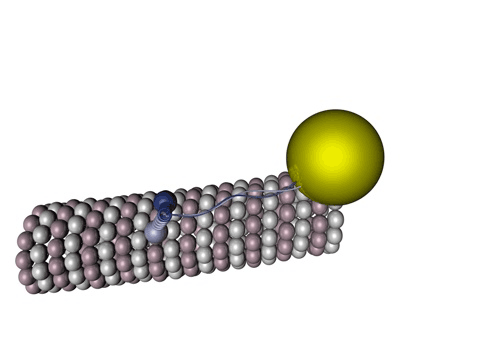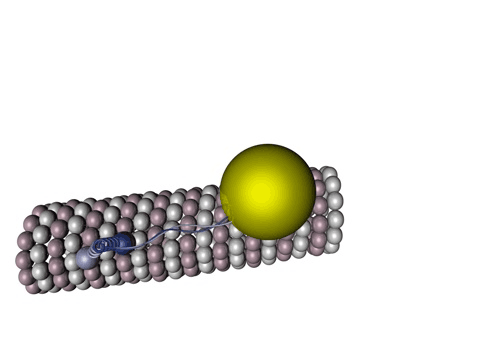
Figure 1 - Kinesin walking on a microtubule.
Source: Wikimedia Commons
Tau protein is a molecule predominantly present in neurons and its functions vary from axonal growth, membrane organization to, mainly, microtubule stabilization. Those microtubules are one of the blocks that sustain the cell’s structures, including its own shape, being present in all living cells: they have a lot of very important functions, from cell division, apoptosis (controlled death of a cell), endocytosis to large pathways for specific functions in a cell (signalizing cascades).
The problem beings when the Tau protein gets misfolded – that is, when it’s not properly produced in the body and ends up with a shape different from what it needs to have, being named the “Tau hypothesis” for Alzheimer’s Disease. Tau protein, when misfolded, can be metabolized incorrectly by enzymes in the cytoplasm, being the most known and common form the (hyper)phosphorylation: after getting phosphorylated, this protein has the ability to aggregate other Tau proteins, and it eventually leads to great neurofibrillary tangles, that are responsible for inflammation, unwanted cell death and other toxic effects.
Another neurofibrillary tangle commonly associated with Alzheimer’s Disease is called amyloid-β, center of the proposed “amyloid-β hypothesis” for the neurodegenerative disease. Its precursor is naturally produced in cells and is excreted to its exterior, where it can misfold into an amyloid-β plaque that can be trapped inside the cell, forming tangles and causing harmful effects as well.
The objective of Das et al was to test the hormone melatonin in vitro for its potential effect of preventing aggregates of Tau protein, observe how it acts regarding the cells’ morphology and biochemistry.
They first prepared full-length Tau protein (a single Tau protein, not entangled) from transgenic bacteria E. coli BL21, purified after cell disruption and then purified. With the purified molecule the group made aggregates with a substance called heparin, being those tubes used for other tests. then they prepared aggregation tubes, with various concentrations of melatonin.
Graphs from fluorescence assays and CD (circular dichroism) spectroscopy showed that melatonin didn’t interfere with the Tau protein in full-length, but it was previously studied that it did interfere with the formation of tangles. Images from electron microscopy showed that melatonin actually broke down the filaments, meaning that the drug only interferes with the toxic tangle formation.
Additionally, results from size-exclusion chromatography showed that the cells treated with melatonin didn’t have the complex structure characteristic from those tangles, it’s actually implied that the drug hindered the formation of those complexes. The sample with Tau protein full-length and the aggregated Tau protein one treated with melatonin weren’t harmful to the cell culture, being another data that proves its efficiency on preventing the generation of toxic tangles.
From scanning electron microscopy images it is quite clear that melatonin actually made the cells go from an either bloated or irregular appearance to smoother shapes: that means that the tubules, that are related closely to Tau protein, could stabilize the cell shape, meaning that the neuron can get back to its healthy state, or close to.
From what was
written here, such discovery may inspire future studies about other drugs
similar to the hormone melatonin for treatment of Alzheimer’s Disease, a
chronic disease that greatly impairs thousands of elderly people around the
globe, so that there may appear a better option of treatment, or perhaps even a cure for it in the near future.
Erick Yudi Maruyama Rodrigues
Source: Das, R., Balmik, A. A., & Chinnathambi, S. (2020). Effect of Melatonin on Tau aggregation and Tau-mediated cell surface morphology. International Journal of Biological Macromolecules, 152, 30–39. https://doi.org/10.1016/j.ijbiomac.2020.01.296

Comentários
Postar um comentário